- Index
- >Laboratoire Moltech-Anjou
- >Publications
- >Publi. CIMI - ChemPhotoChem, fév. 2022
Publication CIMI - ChemPhotoChem, fév. 2022
Le 15 février 2022
Nouvel article de l'équipe CIMI dans ChemPhotoChem:
Reactivity and Mechanistic Issues in the Photocyclization of Dihalostyryl-Naphthalenes towards Halo-[4]helicenes: A Transposition on a Mallory Theme.
Kévin Martin, Caroline Melan, Thomas Cauchy and Narcis Avarvari
Abstract
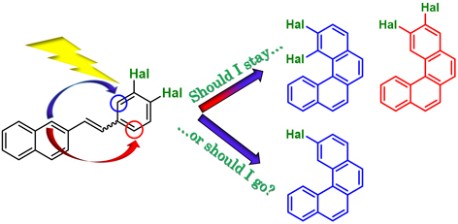 One of the most straightforward strategies nowadays for the synthesis of carbo- and hetero-helicenes is the oxidative photocyclization of stilbene derivatives under Mallory conditions. In this study, the reactivity of a series of 3,4-dihalostyryl-naphthalenes (Hal=Br, F and Cl) has been investigated using Mallory conditions towards the corresponding halogenated [4]helicene compounds. The difluoro precursor afforded the two isomeric 2,3- and 1,2-difluoro-[4]helicenes, resulting from the ring closure on the two possible positions of the substituted benzene ring, the dichloro compound led to the formation of a mixture of the two isomeric 2,3 and 1,2-dichloro-[4]helicenes together with the 2-mono-chloro derivative, while the dibromo precursor provided, unexpectedly, only 2,3-dibromo-[4]helicene and the 2-mono-bromo derivative. DFT calculations performed on the entire series of precursors, final helicenes and intermediate dihydrohelicenes, including the non-halogenated congeners, reveal the highly energetically favorable formation of a second dihydrohelicene intermediate following a [1,9]-hydrogen sigmatropic transposition. Whereas the existence of this transposed dihydrohelicene intermediate, more stable than the initially formed dihydrohelicene by 15–30 kcal/mol, was until now “hidden” in the non-substituted series, it allows herein to explain its re-aromatization through formal elimination of HX in order to provide the 2-mono-halogenated-[4]helicene derivatives.
One of the most straightforward strategies nowadays for the synthesis of carbo- and hetero-helicenes is the oxidative photocyclization of stilbene derivatives under Mallory conditions. In this study, the reactivity of a series of 3,4-dihalostyryl-naphthalenes (Hal=Br, F and Cl) has been investigated using Mallory conditions towards the corresponding halogenated [4]helicene compounds. The difluoro precursor afforded the two isomeric 2,3- and 1,2-difluoro-[4]helicenes, resulting from the ring closure on the two possible positions of the substituted benzene ring, the dichloro compound led to the formation of a mixture of the two isomeric 2,3 and 1,2-dichloro-[4]helicenes together with the 2-mono-chloro derivative, while the dibromo precursor provided, unexpectedly, only 2,3-dibromo-[4]helicene and the 2-mono-bromo derivative. DFT calculations performed on the entire series of precursors, final helicenes and intermediate dihydrohelicenes, including the non-halogenated congeners, reveal the highly energetically favorable formation of a second dihydrohelicene intermediate following a [1,9]-hydrogen sigmatropic transposition. Whereas the existence of this transposed dihydrohelicene intermediate, more stable than the initially formed dihydrohelicene by 15–30 kcal/mol, was until now “hidden” in the non-substituted series, it allows herein to explain its re-aromatization through formal elimination of HX in order to provide the 2-mono-halogenated-[4]helicene derivatives.


